
Sometimes there are very weak attractions between atoms. According to their electron donating or withdrawing abilities, they can form covalent bonds or ionic bonds.

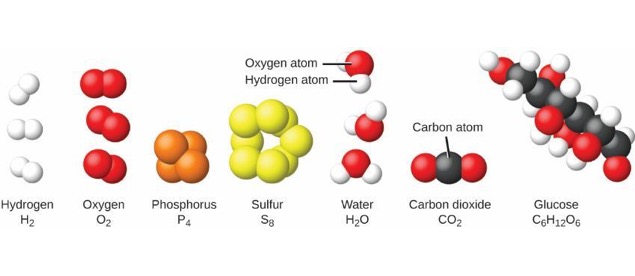
Atoms can join with other atoms in various ways, and form thousands of molecules and other compounds. “Figure 02 01 08” By CNX OpenStax – (CC BY 4.The key difference between element and molecule is that elements are pure substances that consist of only a single type of atom, and cannot be broken down by chemical means while molecules may contain two or more atoms of the same element or different elements.Ītoms are the small units which collect up to form all the existing chemical substances. “207 Ionic Bonding-01” By OpenStax College – Anatomy & Physiology, Connexions Web site. 2017.ġ.”Atom diagram” By The original uploader was Fastfission at English Wikipedia – Transferred from en.wikipedia to Commons by Teratornis using CommonsHelper. 2017.Ģ.”What is molecule? – Definition from .”. However, atoms which don’t have this kind of stability come together to either share or transfer electrons to make molecules and become stable.ġ.”What is an Atom?” LiveScience. The reason for their stability is that they have reached the maximum number of electrons in their outer shells. Only atoms that have noble gas configuration are able to be stable by themselves. Molecules can exist by themselves and are often stable. Atoms can be separated into subatomic particles by nuclear reactions. Molecules can be separated into their constituent atoms by chemical means. This indicates that the chemical properties of atoms are changed when they become molecules. However, table salt (NaCl) is neither a metal nor toxic. For example, Na is a highly reactive metal whereas Cl is a toxic gas. However, chemical properties of atoms are not often retained when they form molecules. As we discussed earlier, atoms make up molecules.
ConclusionĪtoms and molecules are the founding entities of the universe. Molecule: Molecules can be separated into atoms by chemical reactions. Separation is only possible by nuclear reactions. SeparationĪtom:Atoms cannot be separated into subatomic particles by chemical reactions. StabilityĪtom:Atoms are not stable alone, and make chemical bonds with other atoms to become stable. Molecule:Molecules consist of more than two atoms which can be either of the same element or of different elements. ComponentsĪtom: Atoms consist of subatomic particles: protons, electrons, neutrons. They do not display the individual properties of the constituent elements. Molecule:Molecules are made of two or more elements. The smallest singular entity which displays the chemical properties of the corresponding element. The atom cannot be separated into subatomic particles by chemical reactions but the separation is possible by nuclear reactions.įigure 3: Example of Covalent Bonding Read More: Difference Between Covalent and Ionic Bonds Difference Between Atom and MoleculeĪtom:Atoms are the building blocks of molecules. Electrons can be randomly found in pathways called orbitals surrounding the nucleus. The nucleus of an atom is composed of protons which clung together by neutral neutrons to overcome the repulsive forces of like charges. Protons have a positive charge while electrons and neutrons have negative and neutral charges, respectively. The mass of an atom is mainly determined by the protons and neutrons as electrons have negligible mass compared to them. The isotopes differ in their number of neutrons. This combination of subatomic particles is unique for 12C. An atom of a certain element contains a fixed number of electrons, protons, and neutrons most of the time.Ĭarbon atoms contain 6 protons, 6 electrons, and 6 neutrons. Atoms can further be broken down into protons, neutrons, and electrons however, these subatomic particles do not display the chemical characteristics of the element when they are separated. What is the difference between Atom and Molecule What are AtomsĪn atom is defined as the smallest component of an element, which shows the chemical properties that relate to the particular element. – Definition, Structure, Ionic Bonds, Covalent Bondsģ.

The main difference between atom and molecule is their size an atom is the smallest component of an element whereas a molecule is made of two or more atoms. In this article, we will discuss the difference between atom and molecule with regard to their chemical as well as physical properties. Everything around us is made up of either molecules or atoms. Atoms are the building blocks of molecules.


 0 kommentar(er)
0 kommentar(er)
